Perspectiva histórica y nuevas opciones para el control de la mosca de la bichera Cochliomyia hominivorax en Uruguay
DOI:
https://doi.org/10.31285/AGRO.25.974Palabras clave:
biotecnología, CRISPR, impacto económico, ectoparásito, barrenadorResumen
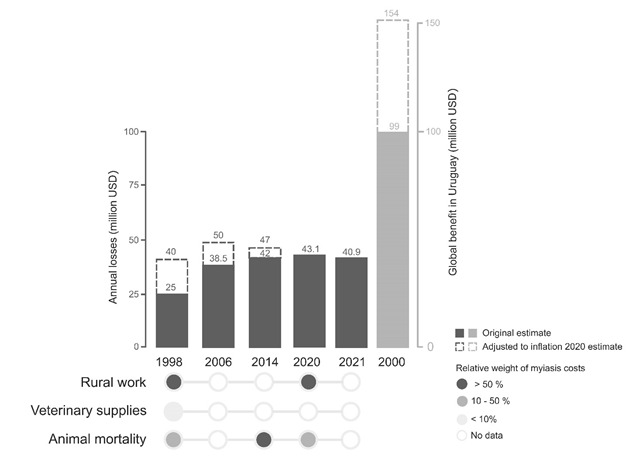
“Mosca de la bichera” o “bichera” son nombres comunes que en Uruguay y la región se da a Cochliomyia hominivorax (Diptera:Calliphoridae), principal agente etiológico de miasis primarias. La miasis ocurre cuando larvas de dípteros infestan animales vivos y al menos durante una parte de su desarrollo se alimentan de los tejidos y fluidos del hospedero. La bichera es un parásito obligatorio y sus tres fases larvarias se desarrollan en los tejidos vivos de hospederos de sangre caliente, incluyendo al hombre. Este comportamiento parasitario causa grandes pérdidas económicas al sector ganadero, además de considerarse un problema desatendido de salud pública. C. hominivorax es endémica de las regiones tropicales y subtropicales de América, pero fue erradicada de América del Norte y Central por un programa, que se extendió por más de 50 años, de Manejo Integrado de Plagas en Áreas Extensas (en inglés AW-IPM) basado en la Técnica del Insecto Estéril (TIE). Desde 2004, se mantiene una barrera en Darién, frontera entre Panamá y Colombia, donde se liberan 14 millones de moscas estériles/semana para evitar su reintroducción. Debido al impacto directo e indirecto en la economía nacional, la compleja logística y el costo del control basado en TIE, Uruguay actualmente está discutiendo estrategias para su erradicación. Las pérdidas directas por miasis en Uruguay oscilan en USD 40-154 millones anuales (2-8% PIB de ganadería). Actualmente, la Organización de las Naciones Unidas para la Alimentación y la Agricultura/Organismo Internacional de Energía Atómica (FAO/OIEA), así como el Departamento de Agricultura de Estados Unidos/Comisión Panamá Estados Unidos para la Erradicación y Prevención del Barrenador del Ganado/Ministerio de Ganadería, Agricultura y Pesca (USDA/COPEG/MGAP) han estado trabajando en propuestas de erradicación para Uruguay. El análisis de costo-beneficio de cada grupo indica un valor neto de aproximadamente USD 98 millones y USD 146 millones, respectivamente, indicando el impacto positivo de la erradicación de la mosca de la bichera para los productores locales, así como para todo el sector ganadero. Sin embargo, una vez alcanzado el estado de libre de miasis en Uruguay, el principal desafío será mantenerlo si Argentina y Brasil no promueven un programa similar, al menos en la región pampeana. Aquí realizamos una revisión bibliográfica, desde el comienzo —durante la década del 40— hasta la erradicación en América del Norte, así como ciclo de vida y parasitismo, y aspectos de genética de poblaciones y ecología. Además, discutimos algunas herramientas biotecnológicas, basadas en transgénesis y edición génica por CRISPR/Cas, que están en desarrollo y prometen transformarse en las nuevas estrategias de control de insectos. Discutimos el balance entre innovación y regulación con base en lecciones aprendidas. Actualmente, en Uruguay se investiga una estrategia de edición génica por CRISPR/Cas para desarrollar un gene drive con financiación de fondos nacionales, lo que garantiza su completo control, e instituciones locales, autoridades y productores pueden ser los propietarios de la biotecnología. Finalmente, el conocimiento generado abre la posibilidad para desarrollar localmente nuevas estrategias biotecnológicas para el control de otros parásitos y/o insectos vectores relevantes en salud animal y pública.
Descargas
Referencias bibliográficas
Ackermann MN, Barboza N, Cicowiez M, Cortelezzi A, Durán V. Evaluación ex-ante de un programa de sanidad animal: un análisis de equilibrio general computable de la erradicación de la mosca de la bichera en Uruguay. La Plata (AR): CEDLAS; 2021. 27p. (Working Papers Nº 289).
Ackermann MN, Barboza N, Cortelezzi A, Durán V. Programa sanitario para erradicar la “bichera”: avances para la evaluación ex ante con un modelo de equilibrio general. In: Anuario de OPYPA 2020. Montevideo: MGAP; 2020. p. 405-16.
Ackermann MN, Cortelezzi A. Empleo en el sector agropecuario 2019 [Internet]. Montevideo: MGAP; 2019 [cited 2021 Sep 25]. Available from: https://bit.ly/3H5aCri.
Altuna M, Hickner PV, Castro G, Mirazo S, Pérez de León AA, Arp AP. New World screwworm (Cochliomyia hominivorax) myiasis in feral swine of Uruguay: one health and transboundary disease implications. Parasit Vectors [Internet]. 2021 [cited 2021 Sep 25];14:26. Doi: 10.1186/s13071-020-04499-z.
Altuna M, Iriarte M, Quevedo M. Muestreo e identificación de larvas de miasis en el departamento de Artigas en el invierno 2015. In: XLIV Jornadas Uruguayas de Buiatría [Internet]. Paysandú: Centro Médico Veterinario de Paysandú; 2016 [cited 2021 Sep 25]. p. 224-6. Available from: https://bit.ly/3BZ73io.
Altuna M, Iriarte MV, Quevedo M. Encuesta sobre miasis en el departamento de Artigas. In: XLIV Jornadas Uruguayas de Buiatría [Internet]. Paysandú: Centro Médico Veterinario de Paysandú, 2016 [cited 2021 Sep 25]. p. 221-3. Available from: https://bit.ly/3bWuAWQ.
Anziani OS, Suarez V. Epidemiología y control de dípteros en estado adulto y larvario en el área central de Argentina. In: Fiel C, Nari A, editors. Enfermedades parasitarias de importancia clínica y productiva en rumiantes. Montevideo: Hemisferio Sur; 2013. p. 552-6.
Araújo H, Carvalho D, Ioshino R, Costa-da-Silva A, Capurro M. Aedes aegypti Control Strategies in Brazil: Incorporation of New Technologies to Overcome the Persistence of Dengue Epidemics. Insects [Internet]. 2015 [cited 2021 Sep 25];6(2):576-94. Doi: 10.3390/insects6020576.
Azeredo-Espin AML. Análise cariotípica, Morfométrica e de Compatibilidade Sexual em linhagens brasileiras de Cochliomyia hominivorax (Diptera: Calliphoridae) [doctoral’s thesis]. Campinas (BR): Universidade Estadual de Campinas; 1987. 149p.
Azeredo-Espin AML. Mitochondrial DNA variability in geographic populations of screwworm fly from Brazil. Int Atom Energy Agency. 1993;327(17):161-5.
Baraldo JD, Durán VD. Evaluación costo beneficio ex ante del programa de erradicación de la mosca de la bichera en Uruguay. Estudios de Economía Agraria y Ambiental. 2021;21(03):27p. Available from: https://bit.ly/3wuua3b.Hernández A, Piaggio J. Situación del GBG al 2015 y antecedentes sobre el impacto socioeconómico de su presencia en el Uruguay. Paper presented at: Reunión Regional para identificar los contenidos del estudio para la determinación del impacto socioeconómico del Gusano Barrenador del Ganado Cochliomyia hominivorax (GBG) en Brasil, Ecuador, Panamá, Paraguay, Perú y Uruguay; 2015 Aug; Asunción, Paraguay.
Barrett WL. Natural dispersion of Cochliomyia americana. J Econ Entomol. 1937;30:873-6.
Barrientos Pontes J, Severo JEV, Garcia EFC, Colares R, Kohek Jr I, Reverbel MS. Projeto demonstrativo de controle e possível erradicação da mosca da bicheira. Hora Vet. 2009;29(171):28-30.
Basmadjián Y, González Arias M, Galiana A, Palma L, González Curbelo M, Acosta G, Rosa R, Gezuele E. Primera notificación de miasis amigdalina humana por Cochliomyia hominivorax (Coquerel, 1858) en Uruguay. In: VIII Jornadas de Zoología del Uruguay [Internet]. Montevideo: Sociedad Zoologica del Uruguay; 2005 [cited 2021 Sep 25]. p. 37. Available from: https://bit.ly/3H6bWKj.
Batista MRD, Ananina G, Azeredo-Espin AML, Klaczko LB. Photographic map of the polytene chromosomes of Cochliomyia hominivorax. Med Vet Entomol. 2009;23(Suppl 1):92-7.
Baumhover A. A Personal Account of Developing the Sterile Insect Technique to Eradicate the Screwworm from Curacao, Florida and the Southeastern United States. Fla Entomol. 2002;85:666-73.
Baumhover AH. Susceptibility of screwworm larvae and prepupae to desiccation. J Econ Entomol. 1963;56:645-9.
Bergamo LW, Fresia P, Azeredo-Espin AM. Incongruent nuclear and mitochondrial genetic structure of new world screwworm fly populations due to positive selection of mutations associated with dimethyl- and diethyl-organophosphates resistance. PLoS One [Internet]. 2015 [cited 2021 Sep 25];10(6):e0128441. Doi: 10.1371/journal.pone.0128441.
Bergamo LW, Fresia P, Azeredo-Espin AML. Phylogeography and insecticide resistance of the New World Screwworm fly in South America and the Caribbean. In: Hendrichs J, Pereira R, Vreysen MJB, editors. Area-Wide Integrated Pest Management. Boca Ratón: CRC Press; 2020. p. 305-17.
Bergamo LW, Fresia P, Lyra ML, Azeredo-Espin AML. High Genetic Diversity and No Population Structure of the New World Screwworm Fly Cochliomyia hominivorax (Diptera: Calliphoridae) on a Microgeographic Scale: Implications for Management Units. J Econ Entomol. 2018;111(5):2476-82.
Bergamo LW, Silva-Brandão KL, Vicentini R, Fresia P, Azeredo-Espin AML. Genetic Differentiation of a New World Screwworm Fly Population from Uruguay Detected by SNPs, Mitochondrial DNA and Microsatellites in Two Consecutive Years. Insects [Internet]. 2020 [cited 2021 Sep 25];11(8):539. Doi: 10.3390%2Finsects11080539.
Brenner R. Distribution of Screwworms (Diptera: Calliphoridae) Relative to Land Use and Topography in the Humid Tropics of Southern Mexico. Ann Entomol Soc Am. 1985;78:433-9.
Burt A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc Biol Sci. 2003;270:921-8.
Bush GL, Neck RW. Ecological genetics of screwworm fly, Cochliomyia hominivorax (Diptera-Calliphoridae) and its bearing on quality-control of mass-reared insects. Environ Entomol. 1976;5(5):821-6.
Bush GL, Neck RW, Barrie G. Screwworm Eradication: Inadvertent Selection for Noncompetitive Ecotypes during Mass Rearing. Science. 1976;193(4252):491-3.
Bushland RC. Eradication program in the Southwestern United States. Misc Publ Entomol Soc Am. 1985;62:12-5.
Carballo M, Colombo A, Heinzen T. Presencia de especies de dípteros califóridos causantes de Miasis cutáneas en el Uruguay: relevamiento de larvas parasitarias (instar III) en rumiantes. Veterinaria. 1990;26(109):4-6.
Carballo M, Heinzen T, Colombo A, Rodríguez M. Datos obtenidos a partir de una encuesta relativo a la incidencia de miasis cutáneas en diferentes zonas del Uruguay. Veterinaria. 1991;28:5-15.
Carvalho RA, Azeredo-Espin AML, Torres TT. Deep sequencing of New World screwworm transcripts to discover genes involved in insecticide resistance. BMC Genomics [Internet]. 2010 [cited 2021 Sep 25];11:695. Doi: 10.1186/1471-2164-11-695.
Carvalho RA, Limia CEG, Bass C, Azeredo-Espin AML. Changes in the frequency of the G137D and W251S mutations in the carboxylesterase E3 gene of Cochliomyia hominivorax (Diptera: Calliphoridae) populations from Uruguay. Vet Parasitol. 2010;170:297-301.
Carvalho RA, Torres TT, Azeredo-Espin AML. A survey of mutations in the Cochliomyia hominivorax (Diptera: Calliphoridae) esterase E3 gene associated with organophosphate resistance and the molecular identification of mutant alleles. Vet Parasitol. 2006;140:344-51.
Carvalho RA, Torres TT, Paniago MG, Azeredo-Espin AML. Molecular characterization of esterase E3 gene associated with organophosphorus insecticide resistance in the New World screwworm fly, Cochliomyia hominivorax. Med Vet Entomol. 2009;23:86-91.
Champer J, Yang E, Lee E, Liu J, Clark AG, Messer PW. A CRISPR homing gene drive targeting a haplolethal gene removes resistance alleles and successfully spreads through a cage population. PNAS. 2020;117(39):24377-83.
Cocke J. New Advances Against the Screwworm. Texas: Texas Agricultural Extension Service; 1981. 11p.
Collins JP. Gene drives in our future: challenges of and opportunities for using a self-sustaining technology in pest and vector management. BMC Proc [Internet]. 2018 [cited 2021 Sep 25];12:9. Doi: 10.1186/s12919-018-0110-4.
Concha C, Palavesam A, Guerrero FD, Sagel A, Li F, Osborne JA, Hernandez Y, Pardo T, Quintero G, Vasquez M, Keller GP, Phillips PL, Welch JB, McMillan WO, Skoda SR, Scott MJ. A transgenic male-only strain of the New World screwworm for an improved control program using the sterile insect technique. BMC Biol [Internet]. 2016 [cited 2021 Sep 25];14:72. Doi: 10.1186/s12915-016-0296-8.
Concha C, Yan Y, Arp A, Quilarque E, Sagel A, Pérez de León A, McMillan WO, Skoda S, Scott MJ. An early female lethal system of the New World screwworm, Cochliomyia hominivorax, for biotechnology-enhanced SIT. BMC Genet [Internet]. 2020 [cited 2021 Sep 25];21(Suppl 2):143. Doi: 10.1186/s12863-020-00948-x.
Coquerel C. Note sur des larves appartenant à une espèce nouvelle de diptère (Lucilia hominivorax) développées dans les sinus frontaux de l’homme à Cayenne. Ann Soc Entomol Fr. 1958;(27):171-6.
Coronado A, Kowalski A. Current status of the New World Screwworm Cochliomyia hominivorax in Venezuela. Med Vet Entomol. 2009;23(Suppl. 1):106-10.
Costa-Júnior LM, Chaves DP, Brito DRB, dos Santos VAF, Costa-Júnior HN, Barros ATM. A review on the occurrence of Cochliomyia hominivorax (Diptera: Calliphoridae) in Brazil. Rev Bras Parasitol Vet. 2019;28(4);548-62.
Crystal MM. Reproductive behavior of laboratory reared screwworm flies (Diptera: Calliphoridae). J Med Entomol. 1967;4:443-50.
Cushing EC, Patton WS. Studies on the higher Diptera of medical and veterinary importance, Cochliomyia hominivorax, sp. nov., the screwworm fly of the New World. Ann trop med parasitol. 1933;27(4):539-51.
Davis EE, Prater T. Economic Impact of the Screwworm Program on the Southwest. Texas: Texas Agricultural Extension Service; 1973.
Deonier CC. Seasonal abundance and distribution of certain blowflies in Southern Arizona and their economic importance. J Econ Entomol. 1942;35:65-70.
Dev V, LaChance LE, Whitten CJ. Polytene chromosomes of the screwworm fly, Cochliomyia hominivorax. J Hered. 1985;76:132-3.
Dev V, Lachance LE, Whitten CJ. Polytene chromosomes, karyotype correlations, and population cytology of the primary screwworm fly. J Hered. 1986;77:427-34.
Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science [Internet]. 2014 [cited 2021 Sep 25];346(6213):1258096. Doi: 10.1126/science.1258096.
Esvelt K. Gene editing can drive science to openness. Nature [Internet]. 2016 [cited 2021 Sep 25];534(7606):153. Doi: 10.1038/534153a.
Esvelt KM, Smidler AL, Catteruccia F, Church GM. Emerging Technology: Concerning RNA-guided gene drives for the alteration of wild populations. eLife [Internet]. 2014 [cited 2021 Sep 25]. Doi: 10.7554/eLife.03401.
FAO. Manual for the control of the Screwworm fly Cochliomyia hominivorax, Coquerel. Roma: FAO; 1990 [cited 2021 Sep 25]. 105p. Available from: https://bit.ly/3BZOH0H.
FAO. Manual para el control de la mosca del gusano barrenador del ganado [Internet]. Vol. 1, Cochliomyia hominivorax (Coquerel). Roma: FAO; 1993 [cited 2021 Sep 25]. 70p. Available from: https://bit.ly/3BYyIQD.
FAO. The New World screwworm eradication programme: North Africa 1988-1992. Rome: FAO; 1992. 192p.
Feyereisen R. Molecular biology of insecticide resistance. Toxicol Lett. 1995;82-83:83-90.
Fresia P, Azeredo-Espin AM, Lyra ML. The phylogeographic history of the new world screwworm fly, inferred by approximate bayesian computation analysis. PLoS One [Internet]. 2013 [cited 2021 Sep 25];8(10):e76168. Doi: 10.1371/journal.pone.0076168.
Fresia P, Lyra ML, Coronado A, Azeredo-Espin AML. Genetic structure and demographic history of New World screwworm across its current geographic range. J Med Entomol. 2011;48:280-90.
Fresia P, Silver M, Mastrangelo T, Azeredo-Espin AML, Lyra ML. Applying spatial analysis of genetic and environmental data to predict connection corridors to the New World screwworm populations in South America. Acta Trop. 2014;138(Suppl.):S34-41.
Fuller G. How screwworm eradication will affect wildlife. Cattlem (Hastings). 1962;48:82-4.
Garcia R, Mendez L, Serrano E, Gil Morales T, Vreysen MJB. Insecticidal wound treatment of livestock on Isla de la Juventud, Cuba: an efficient suppression method of New World Screwworm Cochliomyia hominivorax prior to the release of Sterile Insect. In: Vreysen MJB, Robinson AS, Hendrichs J, editors. Area-wide control of insect pests: from research to field implementation. Dordrecht: Springer; 2007. p. 393-403.
Georghiou GP. Overview of insecticide resistance. In: Green MB, LeBaron HM, Moberg WK, editors. Managing resistance to agrochemicals: from fundamental research to practical strategies. Washington: American Chemical Society; 1990. p. 18-41. (ACS symposium series; 421).
Gil A, Marques L, Pérez Rama R, Piaggio J, Altuna M, Caponi O, Fernández F, Mendoza R. Bichera: resultados y conclusiones de la prueba piloto. Rev Plan Agropecu. 2009;(132):36-9.
González Arias M, Romero S, González M, Galiana A, Basmadjian Y. Miasis en niños hospitalizados en el Centro Hospitalario Pereira Rossell, Uruguay, 2001-2004. In: XIX Congreso Latinoamericano de Parasitología; Asunción, Paraguay; 22 al 24 de octubre de 2009: libro de resúmenes [Interent]. [cited 2021 Sep 25]. p. 257. Available from: https://bit.ly/3D04Dl3.
Goodenough JL, Brown HE, Wendel LE, Tannahill FH. Screwworm eradication program: a review of recent mass-rearing technology. Southwest entomol. 1983;8(1):16-31.
Goodwin JW. An Estimation of Consumer Surplus via Screwworm Eradication in Southwest. Stillwater (OK): Oklahoma State Department of Agriculture Report; 1974.
Grindle J. Economic impact of NWS [New World Screwworm] eradication from North Africa. Rome: FAO; 1991. 33p.
Grupo Técnico de la Dirección General de Servicios Ganaderos del MGAP. Proyecto para erradicar el gusano barrenador en los países del MERCOSUR. In: Anuario OPYPA 2009. Montevideo: MGAP; 2009. p. 391-400.
Guimarães JH, Papavero N, do Prado AP. As miíases na região neotropical (identificação, biologia, bibliografia). Rev bras zool. 1983;1:239-416.
Guimarães JH, Papavero N. Myiasis in man and animals in the neotropical region. Sao Paulo: Plêiade; 1999. 308p.
Gutierrez AP, Ponti L, Arias PA. Deconstructing the eradication of new world screwworm in North America: retrospective analysis and climate warming effects. Med Vet Entomol. 2019;33:282-95.
Gutierrez AP, Ponti L. The New World screwworm: prospective distribution and role of weather in eradication. Agric For Entomol. 2014;16:158-73.
Hall DG. The Blowflies of North America. Baltimore: Thomas Say Foundation; 1948. 477p.
Hall M, Wall R. Myiasis of human and domestic animals. Adv Parasitol. 1995;35:256-333.
Hall MJR, Wall RL, Stevens JR. Traumatic Myiasis: A Neglected Disease in a Changing World. Ann Rev Entomol. 2016;61(1):159-76.
Hall MJR. Screwworm flies as agents of wound myiasis. World animal review [Internet]. 1991 [cited 2021 Sep 25]; Special issue. Available from: https://bit.ly/3EQmhIu.
Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, Gribble M, Baker D, Marois E, Russell S, Burt A, Windbichler N, Crisanti A, Nolan T. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol. 2016;34(1):78-83.
Heidari R, Devonshire AL, Campbell BE, Dorrian SJ, Oakeshott JG, Russell RJ. Hydrolysis of pyrethroids by carboxylesterases from Lucilia cuprina and Drosophila melanogaster with active sites modified by in vitro mutagenesis. Insect Biochem Mol Biol. 2005;35:597-609.
Hemingway J, Field L, Vontas J. An Overview of Insecticide Resistance. Science. 2002;298(5591):96-7.
Hemingway J, Hawkes NJ, McCarroll L, Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol. 2004;34:653-65.
Hendrichs J, Kenmore P, Robinson A, Vreysen M. Area-wide integrated pest management (AW-IPM): principles, practice and prospects. In: Vreysen MJB, Robinson AS, Hendrichs J, editors. Area-wide control of insect pests: from research to field implementation. Dordrecht: Springer; 2007. p. 3-33.
Hendrichs J, Vreysen MJB, Enkerlin WR, Cayol JP. Strategic options in using sterile insects for area-wide integrated pest management. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management [Internet]. 2nd ed. New York: CRC Press; 2021 [cited 2021 Sep 25]. p. 841-84. Doi: 10.1201/9781003035572.
Hightower BG, Adams AL, Alley DA. Dispersal of released irradiated laboratory-reared screw-worm flies. J Econ Entomol. 1965;58(2):373-4.
Hightower BG, Alley DA. Local Distribution of Released Laboratory -reared Screw- worm Flies in Relation to Water Sources. J Econ Entomol. 1963;56(6):798-802.
Hightower BG, Davis RB, Baumhover AH, Graham OH. Seasonal Abundance of the Screw-worm in Northern Mexico. J Econ Entomol. 1966;59(2):416-20.
Hightower BG. Population Dynamics of the Screwworm Fly, Cochliomyia hominivorax (Coquerel), with Respect to Control by the Sterile-male Technique. In: Insect Ecology and the Sterile-Male Technique. Vienna: International Atomic Energy Agency; 1969. p. 25-31.
Holdsworth PA, Kemp D, Green P, Peter RJ, De Bruin C, Jonsson N, Letonja T, Rehbein S, Vercruysse J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guidelines for evaluating the efficacy of acaricides against ticks (Ixodidae) on ruminants. Vet Parasitol. 2006;136:29-43.
Infante-Malachias ME, Yotoko KSC, Azeredo-Espin AML. Random amplified polymorphic DNA of screwworm fly populations (Diptera: Calliphoridae) from southeastern Brazil and northern Argentina. Genome. 1999;42:772-9.
Infante-Malachias ME. Estrutura genética de populações de Cochliomyia hominivorax (Diptera: Calliphoridae) da região sudeste do Brasil: análise através de três tipos de marcadores genéticos [doctoral’s thesis]. Campinas (BR): Universidade Estadual de Campinas; 1999. 122p.
Infante-Vargas ME, Azeredo-Espin AML. Genetic Variability in Mitocondrial DNA of Screwworm Cochliomyia hominivorax (Diptera: Calliphoridae) from Brazil. Biochem Genet. 1995;33:737-56.
Irastorza JM, Bajatta C, Ortega J, Martinez SJ. Erradicación del gusano barrenador del Nuevo Mundo. In: Management of insect pests: nuclear and related molecular and genetic techniques. Proceedings of an International Conference Held in Vienna, Austria, 19–23 October 1992. Vienna: The Agency; 1993. p. 313-8.
Jinkins JE, Davis E, Jones L, Lacewell R. Economic impact from Screw-worm eradication in Mexico. Texas: College Station; 1982. 304p.
Jinkins JE, Davis EE, Jones LL, Lacewell RD. Evaluation of the Mexican-American Screwworm Eradication Programme in Mexico. Vol. 1, Economic Impact from SW Eradication in Mexico. Texas: Texas Agricultural Extension Service; 1985. 196p.
Kaufman G, Wasserman M. Effects of Irradiation on the Screw-worm, Callitroga hominivorax (Coquerel). University of Texas Publications. 1957;5721:246-59.
Klassen W, Curtis CF, Hendrichs J. History of the Sterile Insect Technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management [Internet]. 2nd ed. New York: CRC Press; 2021 [cited 2021 Sep 25]. p. 1-44. Doi: 10.1201/9781003035572.
Klassen W, Curtis CF. History of the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. New York: CRC Press; 2005. p. 3-36.
Klassen W, Vreysen MJB. Area-Wide Integrated Pest Management and the Sterile Insect Technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management [Internet]. 2nd ed. New York: CRC Press; 2021 [cited 2021 Sep 25]. p. 75-112. Doi: 10.1201/9781003035572.
Knipling EF. Possibilities of insect control or eradication through the use of sexually sterile males. J Econ Entomol. 1955;48:459-62.
Knudsen KE, Reid WR, Barbour TM, Bowes LM, Duncan J, Philpott E, Potter S, Scott MJ. Genetic Variation and Potential for Resistance Development to the tTA Overexpression Lethal System in Insects. G3 (Bethesda) [Internet]. 2020 [cited 2021 Sep 25];10(4):1271-81. Doi: 10.1534/g3.120.400990.
Köbrich Grüebler C. Estudio de factibilidad económica para la erradicación de la miasis causada por el gusano barrenador del ganado en América del Sur [Internet]. [place unknown]: OIEA; 2020 [cited 2021 Sep 25]. 58p. Available from: https://bit.ly/3c03H40.
Krafsur ES. Climatological Correlates of Screwworm (Cochliomyia hominivorax) Abundance in Texas, USA. Med Vet Entomol. 1987;1(1):71-80.
Krafsur ES. Screwworm, Cochliomyia hominivorax, Eradication in Texas: Effects of Climate and Strains of Sterile Flies. Entomol Exp Appl. 1985;37(3):297-305.
Krafsur ES, Lindquist DA. Did the sterile insect technique or weather eradicate screwwoms (Diptera: Calliphoridae) from Libya? J Med Entomol. 1996;33:877-87.
Krafsur ES, Whitten CJ. Breeding structure of screwworm fly populations (Diptera-Calliphoridae) in Colima, Mexico. J Med Entomol. 1993;30(2):477-80.
Laake EW. Economic studies of screwworm flies, Cochliomyia species (Diptera: Calliphorinae), with special reference to the myiasis of domestic animals. Iowa state j sci [Internet]. 1935 [cited 2021 Sep 25];345-59. Available from: https://bit.ly/3H6ac3J.
Laake EW. Screw-worm Survey in Western United States, 1949. J Econ Entomol. 1950;43(3):387-9.
Laake EW, Cushing EC, Parish HE. Biology of the primary screwworm fly, Cochliomyia americana, and a comparison of its stages with those of C. macellaria. Washington: USDA; 1936. 24p. (Technical Bulletin; 500).
LaChance LE, Bartlett AC, Bram RA, Gagne RJ, Graham OH, McInnis DO, Whitten CJ, Seawright JA. Mating types in screwworm populations? Science. 1982;218:1142-5.
LaChance LE, Whitten CJ. Cytogenetic studies of screwworm (Diptera: Calliphoridae) populations from southern Mexico and Jamaica. Ann Entomol Soc Am. 1986;79:792-8.
Lessinger AC, Martins Junqueira AC, Lemos TA, Kemper EL, Da Silva FR, Vettore AL, Arruda P, Azeredo-Espin AML. The mitochondrial genome of the primary screwworm fly Cochliomyia hominivorax (Diptera: Calliphoridae). Ins Molec Biol. 2000;9(5):521-9.
Lindquist AW. Myiasis in wild animals in southwestern Texas. J Econ Entomol. 1937;30:735-40.
Lindquist AW. The Use of Gamma Radiation for Control or Eradication of the Screwworm. J Econ Entomol. 1955;48(4):467-9.
Lindquist AW, Barrett WL Jr. Overwintering of Cochliomyia americana at Uvalde, Texas. J Econ Entomol. 1945;38(1):77-83.
Lyra ML. Variabilidade mitocondrial e morfológica em populações naturais da mosca da bicheira, Cochliomyia hominivorax [doctoral’s thesis]. Campinas (BR): Universidade Estadual de Campinas; 2008. 195p.
Lyra ML, Fresia P, Gama S, Cristina J, Klaczko LB, Azeredo-Espin AML. Analysis of Mitochondrial DNA Variability and Genetic Structure in Populations of New World Screwworm Flies (Diptera: Calliphoridae) from Uruguay. J Med Entomol. 2005;42:589-95.
Lyra ML, Klaczko LB, Azeredo-Espin AML. Complex pattern of genetic distribution in populations of the New World screwworm fly revealed by mitochondrial DNA markers. Med Vet Entomol. 2009;23:32-42.
Manchini T, Fulgueiras P, Fente A. Miasis oral: a propósito de un caso. Odontoestomatologia. 2009;11(12):38-43.
Mangan RL, Bouyer J. Population Suppression in Support of the Sterile Insect Technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management [Internet]. 2nd ed. New York: CRC Press; 2021 [cited 2021 Sep 25]. p. 549-74. Doi: 10.1201/9781003035572.
Mangan RL, Thomas DB. Habitat preferences and dispersal patterns in native screwworm fly (Diptera:Calliphoridae). Ann Entomol Soc Am. 1989;82:332-9.
Marburger RG, Thomas JW. A die-off in white-tailed deer of the central mineral region of Texas. J Wildl Manage. 1965;29:706-16.
Marques L, Fernández F, Iriarte V. Estudios epidemiológicos de las miasis cutáneas a C. hominivorax en el Uruguay [Internet]. Montevideo: INIA; 2019 [cited 2021 Sep 25]. 79p. (Serie FPTA; 334). Available from: https://bit.ly/3bVWzpq.
Marshall JM, Hay BA. Confinement of gene drive systems to local populations: a comparative analysis. J Theor Biol. 2012;294:153-71.
Mastrangelo T, Fresia P, Lyra ML, Rodrigues RA, Azeredo-Espin AML. Genetic diversity and population structure of the New World screwworm fly from the Amazon region of Brazil. Acta Trop. 2014;138(Suppl.):S26-33.
Matlock Jr RB, Skoda SR. Mark-recapture estimates of recruitment, survivorship and population growth rate for the screwworm fly, Cochliomyia hominivorax. Med Vet Entomol. 2009;23(Suppl. 1):111-25.
Matlock R, Welch J, Parker F. Estimating Population Density Per Unit Area from Mark, Release, Recapture Data. Ecol Appl. 1996;6(4):1241-53.
Maxwell MJ, Subia J, Abrego J, Garabed R, Xiao N, Toribio RE. Temporal and spatial analysis of the New World screwworm (Cochliomyia hominivorax) in Darien and Embera, Panama (2001–2011). Transbound Emerg Dis. 2017;64:899-905.
Mayer DG, Atzeni MG. Estimation of dispersal distances for Cochliomyia hominivorax (Diptera: Calliphoridae). Environ Entomol. 1993;22:368-74.
McInnis DO. Cytogenetics of a Local Population of the Screwworm, Cochliomyia hominivorax, From Northeastern Mexico. Ann Entomol Soc Am. 1981;74(6):582-9.
McInnis DO, Whitten CJ, Mackley JW, Peterson II RD, Spencer JP. Cytogenetic Studies of the Screwworm, Cochliomyia hominivorax (Diptera: Calliphoridae), from Chiapas, Mexico. Ann Entomol Soc Am. 1983;76(4):628-40.
Melvin R, Bushland RC. A Method of Rearing Cochliomyia Americana C. and P. on Artificial Media. Washington: USDA; 1936. 2p.
Menchaca A, Anegon I, Whitelaw CBA, Baldassarre H, Crispo M. New insights and current tools for genetically engineered (GE) sheep and goats. Theriology. 2016;86(1):160-9.
Miller RS, Sweeney SJ, Slootmaker C, Grear DA, Di Salvo PA, Kiser D, Shwiff SA. Cross-species transmission potential between wild pigs, livestock, poultry, wildlife, and humans: implications for disease risk management in North America. Sci Rep [Internet]. 2017 [cited 2021 Sep 25];7(1):7821. Doi: 10.1038/s41598-017-07336-z.
Ministerio de Ganadería, Agricultura y Pesca, DIEA (UY). Anuario Estadístico Agropecuario 2020. Montevideo: MGAP; 2020. 270p.
Ministerio de Ganadería, Agricultura y Pesca, DIEA (UY). Anuario Estadístico Agropecuario 2006. Montevideo: MGAP; 2006 [cited 2021 Sep 25]. 197p. Available from: https://bit.ly/31EXkRT.
Ministerio de Ganadería, Agricultura y Pesca, DIEA (UY). Anuario Estadístico Agropecuario 2019. Montevideo: MGAP; 2019 [cited 2021 Sep 25]. 255p. Available from: https://bit.ly/3qk2WLA.
Mitchell HJ, Bartsch D. Regulation of GM Organisms for Invasive Species Control. Front Bioeng Biotechnol [Internet]. 2020 [cited 2021 Sep 25];7:454. Doi: 10.3389%2Ffbioe.2019.00454.Wareham C, Nardini C. Policy on synthetic biology: deliberation, probability, and the precautionary paradox. Bioethics. 2015;29(2);118-25.Welch JB. Predation by spiders on ground-released screwworm flies, Cochliomyia hominivorax (Diptera: Calliphoridae) in a mountainous area of southern Mexico. J Arachnol. 1993;21(1):23-8.
Moro D, Byrne M, Kennedy M, Campbell S, Tizard M. Identifying knowledge gaps for gene drive research to control invasive animal species: the next CRISPR step. Glob Ecol Conserv [Internet]. 2018 [cited 2021 Sep 25];13:e00363. Doi: 10.1016/j.gecco.2017.e00363.
Muzzio F, Gil A, Marques L, Pérez Rama R, Piaggio J, Altuna M, Caponi O, Fernández F, Mendoza R. Proyecto para erradicar el Gusano Barrenador en los países del MERCOSUR. In: Anuario OPYPA 2009. Montevideo: MGAP; 2009. p. 391-400.
Newcomb RD, Campbell PM, Russell RJ, Oakeshott JG. cDNA cloning, baculovirus-expression and kinetic properties of the esterase, E3, involved in organophosphorus resistance in Lucilia cuprina. Insect Biochem Mol Biol. 1997;27:15-25.
Notejane M, Zabala C, Ibarra L, Sosa L, Giachetto G. Children hospitalized for myiasis in a reference center in Uruguay. Bol Med Hosp Infant Mex [Internet]. 2021 [cited 2021 Sep 25];78(4):287-92. Doi: 10.24875/bmhim.20000236.
Novy JE. Screwworm control and eradication in the southern United States of America. World animal review [Internet]. 1991 [cited 2021 Sep 25]; Special Issue. Available from: https://bit.ly/3qmeSwo.
OIEA; FAO. Situación del GBG en los países participantes del proyecto del Organismo Internacional de Energía Atómica [Internet]. [place unknown]: OIEA; 2018 [cited 2021 Sep 25]. 37p. Available from: https://bit.ly/3BZ7fOA.
Oliva CF, Vreysen MJ, Dupé S, Lees RS, Gilles JR, Gouagna LC, Chhem R. Current status and future challenges for controlling malaria with the sterile insect technique: technical and social perspectives. Acta Trop [Internet]. 2014 [cited 2021 Sep 25];132(Suppl):S130-9. Doi: 10.1016/j.actatropica.2013.11.019.
Orcellet VM. Sobrevida de estadio pupario de Cochliomyia hominivorax, en laboratorio bajo determinadas condiciones de humedad y temperatura (Santa Fe – Argentina). FAVE Secc Cienc vet. 2005;4:1-2.
Oye KA, Esvelt K, Appleton E, Catteruccia F, Church G, Kuiken T, Lightfoot SB, McNamara J, Smidler A, Collins JP. Biotechnology: regulating gene drives. Science. 2014;345(6197);626-8.
Parker FD, Welch JB, Matlock Jr RB. Influence of habitat, season and attractant on adult behavior of the screwworm (Diptera: Calliphoridae) in a tropical dry zone in Costa Rica. J Econ Entomol. 1993;86:1359-75.
Parman DC. Effect of weather on Cochliomyia americana and a review of methods and economic applications of the study. J Econ Entomol. 1945;38:66-76.
Parman DC, Barrett Jr WL. Ranch Management for Screwworm Prevention and Eradication in Texas and Adjoining States. Washington: USDA; 1941. 11p.
Paulo DF, Junqueira ACM, Arp AP, Vieira AS, Ceballos J, Skoda SR, Pérez-de-León AA, Sagel A, McMillan WO, Scott MJ, Concha C, Azeredo-Espin AML. Disruption of the odorant coreceptor Orco impairs foraging and host finding behaviors in the New World screwworm fly. Sci Rep [Internet]. 2021 [cited 2021 Sep 25];11:11379. Doi: 10.1038/s41598-021-90649-x.
Paulo DF, Williamson ME, Arp AP, Li F, Sagel A, Skoda SR, Sanchez-Gallego J, Vasquez M, Quintero G, Pérez de León AA, Belikoff EJ, Azeredo-Espin AML, McMillan WO, Concha C, Scott MJ. Specific Gene Disruption in the Major Livestock Pests Cochliomyia hominivorax and Lucilia cuprina Using CRISPR/Cas9. G3 (Bethesda) [Internet]. 2019 [cited 2021 Sep 25];9(9):3045-55. Doi: 10.1534/g3.119.400544.
Peneda-Vargas N. Screwworm eradication in Mexico: activities of the Mexico-American Screwworm Commission, 1977–84. In: Graham OH, editor. Symposium on the eradication of the screwworm from the United States and Mexico. College Park (Md): Entomological Society of America; 1985. p. 22-7.
Phillips PL, Welch JB, Kramer M. Seasonal and Spatial and Distributions of Adult Screwworms (Diptera: Calliphoridae) in the Panama Canal Area, Republic of Panama. J Med Entomol. 2004;41:121-9.
Piaggio J, Gil A, Caponi O, Marques L, Perez-Rama R, Altuna M, Fernandez F. Economic losses and costs associated with the presence of screwworm (Cochliomyia hominivorax) in Uruguayan livestock. In: International Symposia on Veterinary Epidemiology and Economics proceedings [Internet]. 2009 [cited 2021 Sep 25]. p. 542. Available from: https://bit.ly/3bZTzbu.
Rahn JJ, Barger GL. Weather Conditions and Screwworm Activity. Agric Meteorol. 1973;11:197-211.
Readshaw JL. Screwworm Eradication: A Grand Delusion? Nature. 1986;320(6061):407-10.
Rendón P, McInnis D, Lance D, Stewart J. Medfly (Diptera:Tephritidae) Genetic Sexing: Large-Scale Field Comparison of Males-Only and Bisexual Sterile Fly Releases in Guatemala. J Econ Entomol. 2004;95(5):1547-53.
Rich KM, Winter-Nelson A, Miller GY. Enhancing economic models for the analysis of animal disease. Rev Sci Tech Off int Epiz. 2005;24(3):847-56.
Richardson R, Ellison J, Averhoff W. Autocidal Control of Screwworms in North America. Science. 1982;215(4531):361-70.
Robinson AS, Vreysen MJB, Hendrichs J, Feldmann U. Enabling technologies to improve area-wide integrated pest management programmes for the control of screwworms. Med Vet Entomol. 2009;23:1-7.
Robinson AS. Genetic Basis of the Sterile Insect Technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management [Internet]. 2nd ed. New York: CRC Press; 2021 [cited 2021 Sep 25]. p. 143-62. Doi: 10.1201/9781003035572.
Rodríguez-Hidalgo R, Tapia-Chiriboga A, Arciniegas S, Vanwambeke SO, Benítez-Ortiz W. Epidemiological analysis of the New World screwworm (Cochliomyia hominivorax) in Ecuador. Transbound Emerg Dis. 2019;66(2):968-77.
Roehrdanz RL. Intraspecific genetic variability in mitochondrial DNA of the screwworm fly (Cochliomyia hominivorax). Biochem Genet. 1989;27:551-69.
Roehrdanz RL, Johnson DA. Mitochondrial DNA variation among geographical populations of the screwworm fly Cochliomyia hominivorax. J Med Entomol. 1988;25:136-41.
Schmidt SM, Belisle M, Frommer WB. The evolving landscape around genome editing in agriculture: many countries have exempted or move to exempt forms of genome editing from GMO regulation of crop plants. EMBO Rep [Internet]. 2020 [cited 2021 Sep 25];21(6):e50680. Doi: 10.15252%2Fembr.202050680.
Scholl P, Colwell D, Cepeda-Palacios R. Myiasis (Muscoidea, Oestroidea). In: Mullen GR, Durden LA, editors. Medical and Veterinary Entomology [Internet]. 3rd ed. London: Academic Press; 2019 [cited 2021 Sep 25]. p. 383-419. Doi: 10.1016/b978-0-12-814043-7.00019-4.
Scott MJ, Benoit JB, Davis RJ, Bailey ST, Varga V, Martinson EO, Hickner PV, Syed Z, Cardoso GA, Torres TT, Weirauch MT, Scholl EH, Phillippy AM, Sagel A, Vasquez M, Quintero G, Skoda SR. Genomic analyses of a livestock pest, the New World screwworm, find potential targets for genetic control programs. Commun Biol [Internet]. 2020 [cited 2021 Sep 25];3(1):424. Doi: 10.1038/s42003-020-01152-4.
Silva NM, Azeredo-Espin AML. Investigation of mutations associated with pyrethroid resistance in populations of the New World Screwworm fly, Cochliomyia hominivorax (Diptera: Calliphoridae). Genet Mol Res. 2009;(8):1067-78.
Skoda SR, Philips PL, Sagel A, Chaudhury MF. Distribution and persistence of sterile screwworms (Diptera: Calliphoridae) released at the Panama-Colombia border. J Econ Entomol. 2017;110:783-9.
Skoda SR, Phillips PL, Welch JB. Screwworm (Diptera: Calliphoridae) in the United States: Response to and elimination of the 2016–2017 outbreak in Florida. J Med Entomol. 2018;55:777-86.
Spradbery P. A Tale of Two Species: Screw-worm Fly. Agricultural Zoology Reviews. 1994;6:1-42.
Strode DD. The Ocala deer herd. Tallahassee: Florida Game and Fresh Water Fish Commission; 1954. 44p.
Suárez VH. Prevalencia y costo de las miasis en el ganado ovino y bovino de la región semiárida pampeana. Boletín de Divulgación Técnica. 2002;73:113-6.
Taylor DB, Peterson II RD. Population genetics and gene variation in primary and secondary screwworm (Diptera: Calliphoridae). Ann Entomol Soc Am. 1994;87:626-33.
Taylor DB, Szalanski AL, Peterson II RD. A polymerase chain reaction - restriction fragment length polymorphism technique for identification of screwworms (Diptera: Calliphoridae). Med Vet Entomol. 1996;10:63-70.
Thomas DB. Age Dependent Susceptibility to Drowning in Pharate Screwworms, Cochliomyia hominivorax (Coquerel). Southwest Entomol. 1986;11(3):161-4.
Thomas DB. Behavioral aspects of screwworm ecology. J Kans Entomol Soc. 1993;66:13-30.
Thomas DB. Survival of the Pupal Stage of the Screwworm, Cochliomyia hominivorax (Coquerel) (Diptera: Calliphoridae) in Subtropical Mexico. J Entomol Sci. 1989;24(3):321-8.
Thomas DB. Time-activity budget of adult screwworm behavior (Diptera: Calliphoridae). J Med Entomol. 1991;28:372-7.
Thomas DB, Mangan RL. Oviposition and wound visiting behavior of the screwworm fly Cochliomyia hominivorax (Coquerel). Ann Entomol Soc Am. 1989;82:526-34.
Torres TT, Azeredo-Espin AML. Population structuring in New World Screwworm populations from the Caribbean: insights from microsatellite data. Med Vet Entomol. 2009;23(1):23-31.
Torres TT, Lyra ML, Fresia P, Azeredo-Espin AML. Assessing Genetic Variation in the New World Screwworm Cochliomyia hominivorax populations from Uruguay. In: Vreysen MJB, Robinson AS, Hendrichs J, editors. Area-wide control of insect pests: from research to field implementation. Dordrecht: Springer; 2007. p. 183-91.
Torres TT. Variabilidade Genética e estrutura de populações de Cochliomyia hominivorax (Diptera: Calliphoridae): uma nova perspectiva através de marcadores microssatélites [doctoral’s thesis]. Campinas (BR): Universidade Estadual de Campinas; 2006. 138p.
Travis VB, Knipling FC, Brody LA. Lateral Migration and Depth of Pupation of the Larvae of the Primary Screwworm Cochliomyia americana C. and P. J Econ Entomol. 1940;33(6):847-50.
USDA. 150 Years of Making History: USDA’s 150th Anniversary. Agric Res [Internet]. 2012 [cited 2021 Sep 25]:10-9. Available from: https://bit.ly/30bssIc.
USDA APHIS Veterinary Services. Historical Economic Impact Estimates of New World Screwworm (NWS) in the United States [Internet]. Washington: USDA; 2016 [cited 2021 Sep 25]. 2p. Available from: https://bit.ly/3HgQv9J.
Vargas-Terán M. Hoja de Ruta para la Supresión y Erradicación Progresiva del Gusano Barrenador del Ganado (GBG) Cochliomyia hominivorax del Continente Americano: Contribuyendo a lograr los Objetivos del Milenio 2030 [Internet]. Viena: Organismo Internacional de Energía Atómica; 2018 [cited 2021 Sep 25]. 77p. Available from: https://bit.ly/30h7qIh.
Vargas-Terán M. Todo lo que usted debe saber sobre la erradicación de la miasis causada por el gusano barrenador del ganado [Internet]. [place unknown]: OIEA; 2020 [cited 2021 Sep 25]. 45p. Available from: https://bit.ly/3bUVZs5.
Vargas-Terán M, Hofmann HC, Tweddle NE. Impact of Screwworm Eradication Programmes Using the Sterile Insect Technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. New York: CRC Press; 2005. p. 629-50.
Vargas-Terán M, Hursey BS, Cunningham EP. Eradication of the screwworm from Libya using the sterile insect technique. Parasitol Today. 1994;10(3):119-22.
Vargas-Terán M, Ortiz Moreno G. Propuesta de un Plan Estratégico Subregional para la Erradicación del Gusano Barrenador del Ganado C. hominivorax en América del Sur. Viena: OIEA; 2018. 85p.
Vargas-Terán M, Spradbery JP, Hofmann HC, Tweddle NE. Impact of Screwworm Eradication Programmes Using the Sterile Insect Technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management [Internet]. 2nd ed. New York: CRC Press; 2021 [cited 2021 Sep 25]. p. 949-78. Doi: 10.1201/9781003035572.
Webber BL, Raghu S, Edwards OR. Opinion: Is CRISPR-based gene drive a biocontrol silver bullet or global conservation threat? PNAS. 2015;112(34):10565-7.
Welch JB. Cochliomyia hominivorax (New World screwworm). In: Invasive Species Compendium [Internet]. Wallingford (UK): CAB International; 2019 [cited 2021 Sep 25]. Available from: https://bit.ly/30e40WH.
Whitten CJ. Use of the enzyme technique to assess the quality of mass-reared sterile screwworm flies. Ann Entomol Soc Am. 1980;73:7-10.
Wyss JH. Screw-worm eradication in the Americas: overview. In: Tan KH, editor. Area-Wide Control of Fruit Flies and Other Insect Pests [Internet]. Penang (MY): Penerbit Universiti Sains Malaysia; 2000 [cited 2021 Sep 25]. p. 79-86. Available from: https://bit.ly/3mUYtwW.
Zumpt F. Myiasis in Man and Animals in the Old World. London: Butterworths; 1965. 267p.
Publicado
Cómo citar
Número
Sección
| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |