Identification and characterization of antimicrobial peptides from Feijoa sellowiana transcriptome
DOI:
https://doi.org/10.31285/AGRO.29.1556Keywords:
AMPs, guayabo del país, gene validation, de novo transcriptome, defensinsAbstract
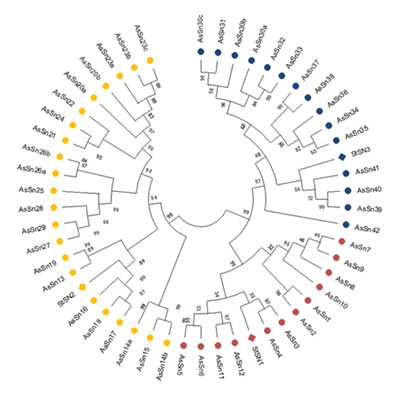
Antimicrobial peptides (AMPs) are small polypeptides present in a wide diversity of phylogenetically distant organisms, including plants. They are part of the innate immune response, allowing rapid action at a lower energetic cost than the adaptive immune system. Various applications of these peptides as anti-infective agents have been demonstrated. Despite their significant potential, the initiatives for bioprospecting of AMPs from the native flora of Uruguay are still incipient. The aim of this study was to identify and characterize genes encoding six antimicrobial peptides families, comprising: defensins, snakins, thionins, hevein-like, lipid transfer proteins (LTPs), and cyclotides in the native fruit species Feijoa sellowiana (Myrtaceae), commonly known as guayabo del país or feijoa. The search was carried out using BLAST, based on a de novo transcriptome of leaf and flower, utilizing reference sequences. The relationships between F. sellowiana and reference protein sequences were characterized through multiple alignments and phylogenetic analysis. The exon-intron structure and isoforms of F. sellowiana AMPs were analyzed by PCR amplification in genomic DNA. This study identified 23 defensins, 49 snakins, 7 thionins, 12 hevein-like and 87 LTPs; no evidence of cyclotide transcripts was found. The expected exon-intron structure was confirmed for five sequences, belonging to defensins, snakins, and hevein-like families.
Downloads
References
Almaghrabi B, Ali MA, Zahoor A, Shah KH, Bohlmann H. Arabidopsis thionin-like genes are involved in resistance against the beet-cyst nematode (Heterodera schachtii). Plant Physiol Biochem. 2019;140:55-67. Doi: 10.1016/j.plaphy.2019.05.005. DOI: https://doi.org/10.1016/j.plaphy.2019.05.005
Apweiler R, Bairoch A, Wu CH, Barker WC, Boeckmann B, Ferro S, Gasteiger E, Huang H, Lopez R, Magrane M, Martin MJ, Natale DA, O'Donovan C, Redaschi N, Yeh LS. UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 2004;32(Database issue):D115-9. Doi: 10.1093/nar/gkh131. DOI: https://doi.org/10.1093/nar/gkh131
Benko-Iseppon AM, Galdino SL, Calsa T Jr, Kido EA, Tossi A, Belarmino LC, Crovella S. Overview on plant antimicrobial peptides. Curr Protein Pept Sci. 2010;11(3):181-8. Doi: 10.2174/138920310791112075. DOI: https://doi.org/10.2174/138920310791112075
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28(1):235-42. Doi: 10.1093/nar/28.1.235. DOI: https://doi.org/10.1093/nar/28.1.235
Berrocal-Lobo M, Segura A, Moreno M, López G, García-Olmedo F, Molina A. Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol. 2002;128(3):951-61. Doi: 10.1104/pp.010685. DOI: https://doi.org/10.1104/pp.010685
Bohlmann H, Broekaert W. The role of Thionins in plant protection. Crit Rev Plant Sci. 1994;13(1):1-16. Doi: 10.1080/07352689409701905. DOI: https://doi.org/10.1080/07352689409701905
Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238-50. Doi: 10.1038/nrmicro1098. DOI: https://doi.org/10.1038/nrmicro1098
Carvalho AO, Gomes VM. Plant defensins--prospects for the biological functions and biotechnological properties. Peptides. 2009;30(5):1007-20. Doi: 10.1016/j.peptides.2009.01.018. DOI: https://doi.org/10.1016/j.peptides.2009.01.018
Castro MS, Gerhardt IR, Orrù S, Pucci P, Bloch C Jr. Purification and characterization of a small (7.3 kDa) putative lipid transfer protein from maize seeds. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794(1):109-14. Doi: 10.1016/s1570-0232(03)00423-9. DOI: https://doi.org/10.1016/S1570-0232(03)00423-9
Craik DJ, Daly NL, Bond T, Waine C. Plant cyclotides: a unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J Mol Biol. 1999;294(5):1327-36. Doi: 10.1006/jmbi.1999.3383. DOI: https://doi.org/10.1006/jmbi.1999.3383
Doyle J. DNA protocols for plants-CTAB total DNA isolation. In: Hewitt GM, Johnston AWB, Young JPW, editors. Molecular techniques in taxonomy. Berlin: Springer; 1991. p. 283-93. Doi: 10.1007/978-3-642-83962-7_18. DOI: https://doi.org/10.1007/978-3-642-83962-7_18
Ganz T. Defensins: antimicrobial peptides of vertebrates. C R Biol. 2004;327(6):539-49. Doi: 10.1016/j.crvi.2003.12.007. DOI: https://doi.org/10.1016/j.crvi.2003.12.007
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40(Database issue):D1178-86. Doi: 10.1093/nar/gkr944. DOI: https://doi.org/10.1093/nar/gkr944
Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95-8.
Joint Genome Institute. Phytozome [Internet]. Berkeley: University of California; 2016-2025 [cited 2025 May 27]. Available from: https://phytozome-next.jgi.doe.gov/
Kaur J, Sagarman US, Shah D. Can plant defensins be used to engineer durable commercially useful fungal resistance in crop plants? Fungal Biol Rev. 2011;25(3):128-35. Doi: 10.1016/j.fbr.2011.07.004. DOI: https://doi.org/10.1016/j.fbr.2011.07.004
Larrañaga P, Diaz-Dellavalle P, Cabrera A, Alem D, Leoni C, Almeida-Souza A, Giovanni-De-Simone S, Dalla-Rizza M. Activity of naturally derived antimicrobial peptides against filamentous fungi relevant for agriculture. Sustain Agric Res. 2012;1(2):211-21. DOI: https://doi.org/10.5539/sar.v1n2p211
Lay FT, Anderson MA. Defensins--components of the innate immune system in plants. Curr Protein Pept Sci. 2005;6(1):85-101. Doi: 10.2174/1389203053027575. DOI: https://doi.org/10.2174/1389203053027575
Li Z, Gao J, Wang G, Wang S, Chen K, Pu W, Wang Y, Xia Q, Fan X. Genome-Wide Identification and Characterization of GASA Gene Family in Nicotiana tabacum. Front Genet. 2022;12:768942. Doi: 10.3389/fgene.2021.768942. DOI: https://doi.org/10.3389/fgene.2021.768942
Nahirñak V, Almasia NI, Hopp HE, Vazquez-Rovere C. Snakin/GASA proteins: involvement in hormone crosstalk and redox homeostasis. Plant Signal Behav. 2012;7(8):1004-8. Doi: 10.4161/psb.20813. DOI: https://doi.org/10.4161/psb.20813
Nahirñak V, Rivarola M, Gonzalez de Urreta M, Paniego N, Hopp H, Almasia N, Vazquez-Rovere C. Genome-wide analysis of the Snakin/GASA gene family in Solanum tuberosum cv. Kennebec. Am J Potato Res. 2016;93(2):172-88. Doi: 10.1007/s12230-016-9494-8. DOI: https://doi.org/10.1007/s12230-016-9494-8
Nawrot R, Barylski J, Nowicki G, Broniarczyk J, Buchwald W, Goździcka-Józefiak A. Plant antimicrobial peptides. Folia Microbiol (Praha). 2014;59(3):181-96. Doi: 10.1007/s12223-013-0280-4. DOI: https://doi.org/10.1007/s12223-013-0280-4
Oberti H, Gutierrez-Gonzalez J, Pritsch C. A first de novo transcriptome assembly of feijoa (Acca sellowiana [Berg] Burret) reveals key genes involved in flavonoid biosynthesis. Plant Genome. 2024;17(3):e20501. Doi: 10.1002/tpg2.20501. DOI: https://doi.org/10.1002/tpg2.20501
Pestana-Calsa MC, Ribeiro IL, Calsa T Jr. Bioinformatics-coupled molecular approaches for unravelling potential antimicrobial peptides coding genes in Brazilian native and crop plant species. Curr Protein Pept Sci. 2010;11(3):199-209. Doi: 10.2174/138920310791112138. DOI: https://doi.org/10.2174/138920310791112138
Pujade-Renaud V, Sanier C, Cambillau L, Pappusamy A, Jones H, Ruengsri N, Tharreau D, Chrestin H, Montoro P, Narangajavana J. Molecular characterization of new members of the Hevea brasiliensis hevein multigene family and analysis of their promoter region in rice. Biochim Biophys Acta. 2005;1727(3):151-61. Doi: 10.1016/j.bbaexp.2004.12.013. DOI: https://doi.org/10.1016/j.bbaexp.2004.12.013
Rivas M, Puppo M, Baccino E, Quezada M, Franco J, Pritsch C. Phenotypic and molecular diversity of wild populations of Acca sellowiana (Berg.) burret in the Southern Area of natural distribution. Horticulturae. 2024;10(4):360. Doi: 10.3390/horticulturae10040360. DOI: https://doi.org/10.3390/horticulturae10040360
Rizwan Z, Aslam N, Zafar F, Humma R, Jamil A. Isolation of novel cyclotide encoding genes from some Solanaceae species and evolutionary link to other families. Pak J Agri Sci. 2021;58(1):169-77. Doi: 10.21162/PAKJAS/21.789.
Rodríguez Decuadro S. Prospección de defensinas y esnaquinas de plantas nativas para el desarrollo de nuevos agentes antimicrobianos [doctoral dissertation]. Montevideo (UY): Universidad de la República, Facultad de Ciencias; 2018. 122p.
Rodríguez-Decuadro S, Barraco-Vega M, Dans PD, Pandolfi V, Benko-Iseppon AM, Cecchetto G. Antimicrobial and structural insights of a new snakin-like peptide isolated from Peltophorum dubium (Fabaceae). Amino Acids. 2018;50(9):1245-59. Doi: 10.1007/s00726-018-2598-3. DOI: https://doi.org/10.1007/s00726-018-2598-3
Rodríguez-Decuadro S, da Rosa G, Radío S, Barraco-Vega M, Benko-Iseppon AM, Dans PD, Smircich P, Cecchetto G. Antimicrobial peptides in the seedling transcriptome of the tree legume Peltophorum dubium. Biochimie. 2021;180:229-42. Doi: 10.1016/j.biochi.2020.11.005. DOI: https://doi.org/10.1016/j.biochi.2020.11.005
Rodríguez-Decuadro S, Dans PD, Borba MA, Benko-Iseppon AM, Cecchetto G. Gene isolation and structural characterization of a legume tree defensin with a broad spectrum of antimicrobial activity. Planta. 2019;250(5):1757-72. Doi: 10.1007/s00425-019-03260-w. DOI: https://doi.org/10.1007/s00425-019-03260-w
Santos-Silva CAD, Ferreira-Neto JRC, Amador VC, Bezerra-Neto JP, Vilela LMB, Binneck E, Rêgo MS, da Silva MD, Mangueira de Melo ALT, da Silva RH, Benko-Iseppon AM. From gene to transcript and peptide: a deep overview on non-specific Lipid Transfer Proteins (nsLTPs). Antibiotics (Basel). 2023;12(5):939. Doi: 10.3390/antibiotics12050939. DOI: https://doi.org/10.3390/antibiotics12050939
Sayers EW, Beck J, Bolton EE, Brister JR, Chan J, Connor R, Feldgarden M, Fine AM, Funk K, Hoffman J, Kannan S, Kelly C, Klimke W, Kim S, Lathrop S, Marchler-Bauer A, Murphy TD, O'Sullivan C, Schmieder E, Skripchenko Y, Stine A, Thibaud-Nissen F, Wang J, Ye J, Zellers E, Schneider VA, Pruitt KD. Database resources of the National Center for Biotechnology Information in 2025. Nucleic Acids Res. 2025;53(D1):D20-D29. Doi: 10.1093/nar/gkae979. DOI: https://doi.org/10.1093/nar/gkae979
Segura A, Moreno M, Madueño F, Molina A, García-Olmedo F. Snakin-1, a peptide from potato that is active against plant pathogens. Mol Plant Microbe Interact. 1999;12(1):16-23. Doi: 10.1094/MPMI.1999.12.1.16. DOI: https://doi.org/10.1094/MPMI.1999.12.1.16
Selitrennikoff CP. Antifungal proteins. Appl Environ Microbiol. 2001;67(7):2883-94. Doi: 10.1128/AEM.67.7.2883-2894.2001. DOI: https://doi.org/10.1128/AEM.67.7.2883-2894.2001
Shang C, Ye T, Zhou Q, Chen P, Li X, Li W, Chen S, Hu Z, Zhang W. Genome-wide identification and bioinformatics analyses of host defense peptides Snakin/GASA in mangrove plants. Genes (Basel). 2023;14(4):923. Doi: 10.3390/genes14040923. DOI: https://doi.org/10.3390/genes14040923
Slavokhotova AA, Shelenkov AA, Korostyleva TV, Rogozhin EA, Melnikova NV, Kudryavtseva AV, Odintsova TI. Defense peptide repertoire of Stellaria media predicted by high throughput next generation sequencing. Biochimie. 2017;135:15-27. Doi: 10.1016/j.biochi.2016.12.017. DOI: https://doi.org/10.1016/j.biochi.2016.12.017
Slavokhotova AA, Shelenkov AA, Odintsova TI. Prediction of Leymus arenarius (L.) antimicrobial peptides based on de novo transcriptome assembly. Plant Mol Biol. 2015;89(3):203-14. Doi: 10.1007/s11103-015-0346-6. DOI: https://doi.org/10.1007/s11103-015-0346-6
Sun B, Zhao X, Gao J, Li J, Xin Y, Zhao Y, Liu Z, Feng H, Tan C. Genome-wide identification and expression analysis of the GASA gene family in Chinese cabbage (Brassica rapa L. ssp. pekinensis). BMC Genomics. 2023;24(1):668. Doi: 10.1186/s12864-023-09773-9. DOI: https://doi.org/10.1186/s12864-023-09773-9
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731-9. Doi: 10.1093/molbev/msr121. DOI: https://doi.org/10.1093/molbev/msr121
Van Damme EJ, Charels D, Roy S, Tierens K, Barre A, Martins JC, Rougé P, Van Leuven F, Does M, Peumans WJ. A gene encoding a hevein-like protein from elderberry fruits is homologous to PR-4 and class V chitinase genes. Plant Physiol. 1999;119(4):1547-56. Doi: 10.1104/pp.119.4.1547. DOI: https://doi.org/10.1104/pp.119.4.1547
Van Parijs J, Broekaert WF, Goldstein IJ, Peumans WJ. Hevein: an antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta. 1991;183(2):258-64. Doi: 10.1007/BF00197797. DOI: https://doi.org/10.1007/BF00197797
Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27-55. Doi: 10.1124/pr.55.1.2. DOI: https://doi.org/10.1124/pr.55.1.2
Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389-95. Doi: 10.1038/415389a. DOI: https://doi.org/10.1038/415389a
Zhu F. Chemical and biological properties of feijoa (Acca sellowiana). Trends Food Sci Technol. 2018;81:121-31. Doi: 10.1016/j.tifs.2018.09.008. DOI: https://doi.org/10.1016/j.tifs.2018.09.008
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Agrociencia Uruguay

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |