Alternatives for replacing or reducing the content of added sulfites in Tannat red wines elaborated with commercial yeasts or with minimal intervention
DOI:
https://doi.org/10.31285/AGRO.29.1590Abstract
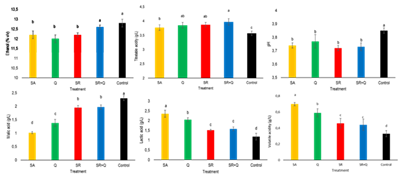
Sulfite reduction in wines represents a significant challenge in the current wine industry given its widespread use as an additive and the potential health risks for consumers. This study evaluates alternatives to reduce sulfites in Tannat red wines made with minimal intervention or with selected yeasts, focusing on microbiological stability, color, and physicochemical composition. In the 2023 vintage, vinifications were carried out with native yeasts and selected yeasts using reduced SO2 (SR: 30 mg/L), chitosan (Q: 100 mg/L), combinations of SO2 and chitosan (SR+Q), lysozyme (SR+L: 5 mg/L) (SR+QL), and fumaric acid (SR+AF: 6 mg/L) that were compared with a Control (125 mg/L of sulfites) and a treatment without additives (SA). Microbial counts in minimal intervention wines and those obtained by sulfite substitution or reduction did not show differences during fermentation. The minimal intervention fermentations rates were slower than the treatments with selected yeasts. From the minimal intervention treatments, the SR+Q wines showed higher malic acid content, color intensity, phenolic compounds, anthocyanins, and tannins compared to the other treatments and similar to the Control. On the other hand, the SR+Q wines from the sulfite substitution or reduction trial also presented values of color intensity and polyphenolic and anthocyanin content similar to the Control. Consequently, the combination of reduced doses of sulfites and chitosan seems to be a viable option for producing Tannat wines with characteristics similar to those made with conventional doses of sulfites, at least, when grape soundness is good, as in the vintage analyzed in the present study.
Downloads
References
Alonso González P, Parga Dans E, Fuentes Fernández R. Certification of natural wine: policy controver-sies and future prospects. Front Sustain Food S. 2022;6:875427. Doi: 10.3389/fsufs.2022.875427. DOI: https://doi.org/10.3389/fsufs.2022.875427
Ayala F, Echávarri JF, Negueruela AI. A new simplified method for measuring the color of wines: I. Red and Rosé Wines. Am J Enol Vitic. 1997;48:357-63. Doi: 10.5344/ajev.1997.48.3.357. DOI: https://doi.org/10.5344/ajev.1997.48.3.357
Ayala F, Echávarri JF, Negueruela AI. MSCVes.zip [Software]. Zaragoza: Universidad de Zaragoza; 2021[cited 2024 Dec 31]. Available from: http://www.unizar.es/negueruela/MSCV.es
Bağder Elmaci S, Gülgör G, Tokatli M, Erten H, İşci A, Özçelik F. Effectiveness of chitosan against wine-related microorganisms. Antonie Van Leeuwenhoek. 2015;107(3):675-86. Doi: 10.1007/s10482-014-0362-6. DOI: https://doi.org/10.1007/s10482-014-0362-6
Bartowsky EJ, Costello PJ, Villa A, Henschke PA. The chemical and sensorial effects of lysozyme addition to red and white wines over six months' cellar storage. Aust J Grape Wine Res. 2004;10(2):143-50. Doi: 10.1111/j.1755-0238.2004.tb00017.x. DOI: https://doi.org/10.1111/j.1755-0238.2004.tb00017.x
Bisson LF. Stuck and sluggish fermentations. Am J Enol Vitic. 1999;50(1):107-19. Doi: 10.5344/ajev.1999.50.1.107. DOI: https://doi.org/10.5344/ajev.1999.50.1.107
Bučková M, Puškárová A, Ženišová K, Kraková L, Piknová Ľ, Kuchta T, Pangallo D. Novel insights into microbial community dynamics during the fermentation of Central European ice wine. Int J Food Microbiol. 2017;266:42-51. Doi: 10.1016/j.ijfoodmicro.2017.11.010. DOI: https://doi.org/10.1016/j.ijfoodmicro.2017.11.010
Costanigro M, Appleby C, Menke SD. The wine headache: consumer perceptions of sulfites and willing-ness to pay for non-sulfited wines. Food Qual Pref. 2014;31:81-9. Doi: 1365-2222.2009.033624. DOI: https://doi.org/10.1016/j.foodqual.2013.08.002
Cuijvers K, Van Den Heuvel S, Varela C, Rullo M, Solomon M, Schmidt S, Borneman A. Alterations in yeast species composition of uninoculated wine ferments by the addition of sulphur dioxide. Fermentation. 2020;6(2):62. Doi: 10.3390/fermentation6020062. DOI: https://doi.org/10.3390/fermentation6020062
Cureau N, Threlfall R, Carbonero F, Howard L, Lavefve L. Fungal diversity and dynamics during grape wine fermentations with different sulfite levels and yeast inoculations. Am J Enol Vitic. 2021;72(3):240-56. Doi: 10.5344/ajev.2021.20054. DOI: https://doi.org/10.5344/ajev.2021.20054
De Revel G, Capela AB, Hogg T. A pre‐spoilage marker for bacterial activity in fortified wine, conversion of L‐malic acid to L‐lactic acid. Lett Appl Microbiol. 1994;18(6):329-32. Doi: 10.1111/j.1472-765X.1994.tb00881.x. DOI: https://doi.org/10.1111/j.1472-765X.1994.tb00881.x
Delfini C, Cersosimo M, Del Prete V, Strano M, Gaetano G, Pagliara A, Ambrò S. Resistance screening essay of wine lactic acid bacteria on lysozyme: efficacy of lysozyme in unclarified grape musts. J Agricul Food Chem. 2004;52(7):1861-6. Doi: 10.1021/jf034824m. DOI: https://doi.org/10.1021/jf034824m
Dias R, Vilas-Boas E, Campos FM, Hogg T, Couto JA. Activity of lysozyme on lactobacillus hilgardii strains isolated from port wine. Food Microbiol. 2015;49:6-11. Doi: 10.1016/j.fm.2015.01.007. DOI: https://doi.org/10.1016/j.fm.2015.01.007
Divol B, du Toit M, Duckitt E. Surviving in the presence of sulphur dioxide: strategies developed by wine yeasts. Applied Microbiol Biotechnol. 2012;95:601-13. Doi: 10.1007/s00253-012-4186-x. DOI: https://doi.org/10.1007/s00253-012-4186-x
Fleet GH. Wine yeasts for the future. FEMS Yeast Res. 2008;8(7):979-95. Doi: 10.1111/j.1567-1364.2008.00427.x. DOI: https://doi.org/10.1111/j.1567-1364.2008.00427.x
Fuentes-Fernandez R. Finding common ground: the need for cooperation and collaboration in the spanish natural wine industry. Wine Bus Case Res J. 2019;3(1):65-93. DOI: https://doi.org/10.26813/wbcrj/2019.03.01/finding
Galati A, Schifani G, Crescimanno M, Migliore G. “Natural wine” consumers and interest in label infor-mation: An analysis of willingness to pay in a new Italian wine market segment. J Clean Prod. 2019; 227:405-13. Doi: 10.1016/j.jclepro.2019.04.219. DOI: https://doi.org/10.1016/j.jclepro.2019.04.219
Gao Y, Tian Y, Liu D, Li Z, Zhang XX, Li JM, Huang JH, Wang J, Pan QH. Evolution of phenolic compounds and sensory in bottled red wines and their co-development. Food Chem. 2002;172:565-74. Doi: 10.1016/j.foodchem.2014.09.115. DOI: https://doi.org/10.1016/j.foodchem.2014.09.115
Glories Y. La couleur des vins rouges: 2e partie: mesure, origine et interprétation. OENO One. 1984;18(4):253-71. Doi: 10.20870/oeno-one.1984.18.4.1744. DOI: https://doi.org/10.20870/oeno-one.1984.18.4.1744
Glories Y. La couleur des vins rouges: lre partie: les équilibres des anthocyanes et des tanins. OENO One.1984;18(3):195-217. Doi: 10.20870/oeno-one.1984.18.3.1751. DOI: https://doi.org/10.20870/oeno-one.1984.18.3.1751
Gómez-Rivas L, Escudero-Abarca BI, Aguilar-Uscanga MG, Hayward-Jones PM, Mendoza P, Ramírez M. Selective antimicrobial action of chitosan against spoilage yeasts in mixed culture fermentations. J Ind Microbiol Biotechnol. 2004;31(1):16-22. Doi: 10.1007/s10295-004-0112-2. DOI: https://doi.org/10.1007/s10295-004-0112-2
Grangeteau C, Roullier‐Gall C, Rousseaux S, Gougeon RD, Schmitt‐Kopplin P, Alexandre H, Guilloux‐Benatier M. Wine microbiology is driven by vineyard and winery anthropogenic factors. Microbial biotechnol. 2017;10(2):354-70. Doi: 10.1111/1751-7915.12428. DOI: https://doi.org/10.1111/1751-7915.12428
Guerrero RF, Cantos-Villar E. Demonstrating the efficiency of sulphur dioxide replacements in wine: a parameter review. Trends Food Sci Technol. 2015;42(1):27-43. Doi: 10.1016/j.tifs.2014.11.004. DOI: https://doi.org/10.1016/j.tifs.2014.11.004
He F, Liang NN, Mu L, Pan QH, Wang J, Reeves MJ, Duan CQ. Anthocyanins and their variation in red wines: I. Monomeric anthocyanins and their color expression. Molecules. 2012;17(2):1571-601. Doi: 10.3390/molecules17021571. DOI: https://doi.org/10.3390/molecules17021571
He F, Liang NN, Mu L, Pan QH, Wang J, Reeves MJ, Duan CQ. Anthocyanins and their variation in red wines: II. Anthocyanin derived pigments and their color evolution. Molecules. 2012;17:1483-519. Doi: 10.3390/molecules17021483. DOI: https://doi.org/10.3390/molecules17021483
Hu S. Natural Wine: the Conundrum [Internet] 2020 [cited 2024 Dec 31]. 16p. Available from: https://shenglihu.com/research/D6_July2020_Shengli_Hu.pdf
International Organisation of Vine and Wine. International Code of Oenological Practices. Paris: OIV; 2021. 435p.
L'Association des Vins Naturels. Cahier des charges d’un vin AVN [Internet]. 2018 [cited 2024 Dec 31]. Available from: http://avn.vin/category/L-association-Cahier-des-charges
Liang M, Liu R, Qi W, Su R, Yu Y, Wang L, He Z. Interaction between lysozyme and procyanidin: multilevel structural nature and effect of carbohydrates. Food Chem. 2013;138(2-3):1596-603. Doi: 10.1016/j.foodchem.2012.11.027. DOI: https://doi.org/10.1016/j.foodchem.2012.11.027
Lopez I, Santamaria P, Tenorio C, Garijo P, Gutierrez AR, Lopez R. Evaluation of lysozyme to control vinification process and histamine production in Rioja wines. J Microbiol Biotechnol. 2009;19(9):1005-12. Doi: 10.4014/jmb.0811.602. DOI: https://doi.org/10.4014/jmb.0811.602
Mas A, Portillo MC. Strategies for microbiological control of the alcoholic fermentation in wines by exploiting the microbial terroir complexity: a mini-review. Int J Food Microbiol. 2022;367:109592. Doi: 10.1016/j.ijfoodmicro.2022.109592. DOI: https://doi.org/10.1016/j.ijfoodmicro.2022.109592
Morata A, Adell E, López C, Palomero F, Suárez E, Pedrero S, Bañuelos MA, González C. Use of fumaric acid to inhibit malolactic fermentation in bottled rioja wines: effect in pH and volatile acidity control. Beverages. 2023;9:16. Doi: 10.3390/beverages9010016. DOI: https://doi.org/10.3390/beverages9010016
Morata A, Bañuelos MA, López C, Song C, Vejarano R, Loira I, Palomero F, Suarez Lepe JA. Use of fumaric acid to control pH and inhibit malolactic fermentation in wines. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2020;37(2):228-38. Doi: 10.1080/19440049.2019.1684574. DOI: https://doi.org/10.1080/19440049.2019.1684574
Morata A, Escott C, Loira I, Del Fresno JM, González C, Suárez-Lepe JA. Influence of Saccharomyces and non-Saccharomyces yeasts in the formation of pyranoanthocyanins and polymeric pigments during red wine making. Molecules. 2019;24:4490. Doi: 10.3390/molecules24244490. DOI: https://doi.org/10.3390/molecules24244490
Navarro Y, Torija MJ, Mas A, Beltran G. Viability-PCR allows monitoring yeast population dynamics in mixed fermentations including viable but non-culturable yeasts. Foods. 2020;9(10):1373. Doi: 10.3390/foods9101373. DOI: https://doi.org/10.3390/foods9101373
Nunes C, Maricato É, Cunha Â, Rocha MA, Santos S, Ferreira P, Silva M, Rodrigues A, Amado O, Coimbra J, Silva D. Chitosan–genipin film, a sustainable methodology for wine preservation. Green Chem. 2016;18(19):5331-41. Doi: 10.1039/C6GC01621A. DOI: https://doi.org/10.1039/C6GC01621A
Payan C. Acidification of musts and wines by fumaric acid: impact of its addition on the chemical and sensory characteristics of varietal wines in the frame of climate change changement climatique [doctoral dissertation]. [place unknown]: Université de Bordeaux; Hochschule Geisenheim University; 2023. 244p.
Picariello L, Errichiello F, Coppola F, Rinaldi A, Moio L, Gambuti A. Effect of chitosan addition on acetaldehyde and polymeric pigments production after oxidation of red wines with different tannin/anthocyanins ratio. Eur Food Res Technol. 2023;249(9):2447-55. Doi: 10.1007/s00217-023-04292-z. DOI: https://doi.org/10.1007/s00217-023-04292-z
Ribéreau-Gayon P, Dubourdieu D, Donèche B, Lonvaud A. Handbook of enology. Vol. 1, The microbiology of wine and vinifications. New Jersey: John Wiley & Sons; 2006. 497p. DOI: https://doi.org/10.1002/0470010363
Ribéreau-Gayon P, Stonestreet E. Le dosage des anthocyanes dans le vin rouge. Bull Soc Chim Fr. 1965;9:2649-52.
Roldán A, Lasanta C, Caro I, Palacios V. Effect of lysozyme on “flor” velum yeasts in the biological aging of sherry wines. Food Microbiol. 2012;30(1):245-52. Doi: 10.1016/j.fm.2011.10.010. DOI: https://doi.org/10.1016/j.fm.2011.10.010
Santos MC, Nunes C, Saraiva JÁ, Coimbra MA. Chemical and physical methodologies for the replace-ment/reduction of sulfur dioxide use during winemaking: review of their potentialities and limitations. Eur Food Res Technol. 2012,234:1-12. Doi: 10.1007/s00217-011-1614-6. DOI: https://doi.org/10.1007/s00217-011-1614-6
Sarneckis C, Dambergs RG, Jones P, Mercurio MD, Herderich MJ, Smith PA. Quantification of condensed tannins by precipitation with methyl cellulose: development and validation of an optimised tool for grape and wine analysis. Aust J Grape Wine Res. 2006;12:39-49. Doi: 10.1111/j.1755-0238.2006.tb00042.x. DOI: https://doi.org/10.1111/j.1755-0238.2006.tb00042.x
Scientific Opinion on the re-evaluation of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228) as food additives. EFSA J. 2016;14(4):4438. Doi: 10.2903/j.efsa.2016.4438. DOI: https://doi.org/10.2903/j.efsa.2016.4438
Sellers-Rubio R, Nicolau-Gonzalbez JL. Estimating the willingness to pay for a sustainable wine using a heckit model. Wine Econ Policy. 2016;5:96-104. Doi: 10.1016/j.wep.2016.09.002. DOI: https://doi.org/10.1016/j.wep.2016.09.002
Singleton V, Rossi J. Colorimetry of total phenolic compounds with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144-58. Doi: 10.5344/ajev.1965.16.3.144. DOI: https://doi.org/10.5344/ajev.1965.16.3.144
Valera MJ, Sainz F, Mas A, Torija MJ. Effect of chitosan and SO2 on viability of Acetobacter strains in wine. Int J Food Microbiol. 2017;246:1-4. Doi: 10.1016/j.ijfoodmicro.2017.01.022. DOI: https://doi.org/10.1016/j.ijfoodmicro.2017.01.022
Vally H, Misso NLA, Madan V. Clinical effects of sulphite additives. Clin Exp Allergy. 2009,39:1643-51. Doi: 1365-2222.2009.03362. DOI: https://doi.org/10.1111/j.1365-2222.2009.03362.x
Varela C, Pizarro F, Agosin E. Biomass content governs fermentation rate in nitrogen-deficient wine musts. Appl Environ Microbiol. 2004;70(6):3392-400. Doi: 10.1128/AEM.70.6.3392-3400.2004. DOI: https://doi.org/10.1128/AEM.70.6.3392-3400.2004
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Agrociencia Uruguay

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |