Sugarcane production and nutrient accumulation in commercial plantations under vinasse irrigation
DOI:
https://doi.org/10.31285/AGRO.29.1468Keywords:
ethanol production, nutrient use efficiency, luxury consumption of K, UruguayAbstract
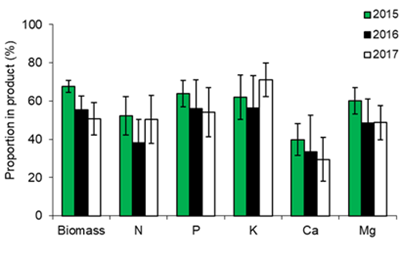
Vinasse is a liquid byproduct of ethanol production used as fertilizer for sugarcane (Saccharum officinarum L). Although the nutrient concentration of vinasse is relatively low, it is considered a valuable source of nutrients. Currently, in Uruguay undiluted vinasse is applied in fields close to the ethanol industrial facilities (ALUR, Bella Union), but its effect on soil and crops has been scarcely studied. This work, carried out between 2014 and 2017, evaluated the production and absorption of nutrients in 21 commercial plantations receiving vinasse (doses between 26.5 and 150 m3 ha-1) to assess its contribution to the crop nutrition and sustainability of the production system. In each plantation the production of biomass and content of nutrients were analyzed (N, P, K, Ca, Mg, Na, Cu, Fe, Mn, and Zn). The yield varied from 34.4 to 125.3 Mg ha-1, (average 64.7 Mg ha-1), while average aerial biomass production was 33.1 Mg ha-1 of dry matter. The highest accumulation of nutrients for the crop corresponded to K (average 216, 154 and 157 kg ha-1 in 2015, 2016 and 2017, respectively), which highlights the importance of vinasse as a K source. However, in some fields a low efficiency of K utilization was estimated. It is concluded that the application of vinasse makes an important contribution of nutrients (N, P and K) to the crop, which allows achieving high yields, as well as saving commercial fertilizers.
Downloads
References
Bremner JM, Mulvaney CS. Nitrogen-Total. In: Page AL, Miller RH, Keeney DR, editors. Methods of soil analyses. Part 2, Chemical and microbiological properties. Madison: SAS; 1982. p. 595-624. DOI: https://doi.org/10.2134/agronmonogr9.2.2ed.c31
Brito FDL, Rolim MM, Pedrosa EM. Efeito da aplicação de vinhaça nas características químicas de três solos da zona da mata canavieira de Pernambuco. Rev Bras Cienc Agrar. 2015;4(4):456-62. DOI: https://doi.org/10.5039/agraria.v4i4a14
Cerri CC, Galdos MV, Maia SMF, Bernoux M, Feigl BJ, Powlson D, Cerri CEP. Effect of sugarcane harvesting systems on soil carbon stocks in Brazil: an examination of existing data. Eur J Soil Sci. 2011;62(1):23-8. DOI: https://doi.org/10.1111/j.1365-2389.2010.01315.x
Cherubin MR, Lisboa IP, Silva AG, Varanda LL, Bordonal RO, Carvalho JL, Otto R, Pavinato OS, Soltangheisi A, Cerri CE. Sugarcane straw removal: implications to soil fertility and fertilizer demand in Brazil. Bioenergy Res. 2019;12(4):888-900. DOI: https://doi.org/10.1007/s12155-019-10021-w
Coale FJ, Sanchez CA, Izuno FT, Bottcher AB. Nutrient accumulation and removal by sugar cane grown on everglades histosols. Agron J. 1993;85:310-15. DOI: https://doi.org/10.2134/agronj1993.00021962008500020028x
Contreras AM, Rosa E, Pérez M, Van Langenhove H, Dewolf J. Comparative life cycle assessment of four alternatives for using by – products for cane sugar production. J Clean Prod. 2009;17:772-9. DOI: https://doi.org/10.1016/j.jclepro.2008.12.001
da Silva A, Rossetto R, Bombecini J, Piemonte M, Muraoka T. Nitrogen mineralization from sugarcane vinasse. J Plant Nutr. 2014;37:1227-36. DOI: https://doi.org/10.1080/01904167.2014.888739
da Silva VSG, de Oliveira MW, Ferreira VM, Oliveira TBA, de Brito Santana M, Galvão ER. Stalk yield and nutrients accumulation of sugarcane varieties in three crop cycles. Rev Bras Cienc Agrar. 2018;41(2):415-23. DOI: https://doi.org/10.19084/RCA17051
de Aquino GS, de Conti Medina C, Junior AD, Pasini A, Brito OR, Cunha AC, dos Santos Junior JH, Kussaba DAO, Almeida LF. Impact of harvesting with burning and management of straw on the industrial quality and productivity of sugarcane. Afri J Agric Res. 2016;11:2462-8. DOI: https://doi.org/10.5897/AJAR2016.11014
de Oliveira Bordonal R, Carvalho JLN, Lal R, de Figueiredo EB, de Oliveira BG, La Scala N. Sustainability of sugarcane production in Brazil: a review. Agron Sustain Dev. 2018;38(2):13. Doi: 10.1007/s13593-018-0490-x. DOI: https://doi.org/10.1007/s13593-018-0490-x
del Pino A, Casanova O, Hernandez J, Takata V, Panissa G. Efecto de la aplicación de vinaza en suelos bajo cultivo de caña de azúcar. Cienc Agronom. 2017;30:30-6.
del Pino A, Casanova O, Hernandez J, Takata V, Panissa G. Vinasse for sugarcane crop nutrition: accumulation and efficiency in the use of nutrients. Austr J Crop Sci. 2022;16(9):1107-16. Doi: 10.21475/ajcs.22.16.09.p3617. DOI: https://doi.org/10.21475/ajcs.22.16.09.p3617
Digonzelli PA, De Ullivarri JF, Medina M, Tortora L, Romero ER, Quinteros HR. Dynamics of sugarcane harvest residue decomposition in Argentina. Proc Int Soc Sugar Cane Technol. 2013;28:1-12.
Durán A, García Préchac F. Suelos del Uruguay. Vol 1, factores ambientales, clasificación y caracterización. Montevideo: Hemisferio Sur; 2009. 74p.
Isaac RA, Kerber JD. Atomic Absorption and flame photometry: techniques and uses in soil, plant and water analysis. In: Walsh LM, editor. Instrumental methods for analysis of soil and plant tissues. Madison: SSAS; 1971. p. 17-37. DOI: https://doi.org/10.2136/1971.instrumentalmethods.c2
Kingston G, Anink MC, Clift BM, Beattie RN. Potassium management for sugarcane on base saturated soils in northern New South Wales. Proc Austr Soc Sugar Cane Technol. 2009;31:186-94.
Leite JM, Ciampitti IA, Mariano E, Vieira-Megda MX, Trivelin PC. Nutrient partitioning and stoichiometry in unburnt sugarcane ratoon at varying yield levels. Front Plant Sci. 2016;7:466. Doi: 10.3389/fpls.2016.00466. DOI: https://doi.org/10.3389/fpls.2016.00466
Lisboa IP, Cherubin MR, Lima RP, Cerri CC, Satiro LS, Wienhold BJ, Schmer MR, Jin VL, Cerri CE. Sugarcane straw removal effects on plant growth and stalk yield. Ind Crops Prod. 2018;111:794-806. DOI: https://doi.org/10.1016/j.indcrop.2017.11.049
Lourenço KS, Suleiman AKA, Pijl A, van Veen JA, Cantarella H, Kuramae EE. Resilience of the resident soil microbiome to organic and inorganic amendment disturbances and to temporary bacterial invasion. Microbiome. 2018;6(1):142. Doi: 10.1186/s40168-018-0525-1. DOI: https://doi.org/10.1186/s40168-018-0525-1
Machado CT, Furlani A, Cangiani M, Machado AT. Índices de eficiência de variedades locais e melhoradas de milho ao fósforo. Bragantia. 2001;60(3):225-38. DOI: https://doi.org/10.1590/S0006-87052001000300010
Meyer J. Sugarcane nutrition and fertilization. In: Meyer JH, Turner PE, Rein P, Mathias K, editors. Good management practices for the cane industry edition. Berlin: Bartens; 2013. p 133-80.
Murphy J, Riley JP. A modified single solution method for determination of phosphate in natural waters. Anal Chem Act. 1962;27:31-6. DOI: https://doi.org/10.1016/S0003-2670(00)88444-5
Nicochelli LM, Nascentes R, Lima EB, Soares FSC. Sorção de potássio em amostras de solo submetidas à aplicação de vinhaça. Rev Bras Engen Agri Amb. 2012;16(7):754-60. DOI: https://doi.org/10.1590/S1415-43662012000700008
Oliveira EC, Freire FJ, Oliveira RID, Freire MB, Simões Neto DE, Silva SA. Nutrient extraction and export by fully irrigated sugarcane varieties. Rev Bras Cienc Solo. 2010;34(4):1343-52. DOI: https://doi.org/10.1590/S0100-06832010000400031
Ortegón GP, Arboleda FM, Candela L, Tamoh K, Valdes-Abellan J. Vinasse application to sugar cane fields: effect on the unsaturated zone and groundwater at Valle del Cauca (Colombia). Sci Total Environ. 2016;539:410-9. Doi: 10.1016/j.scitotenv.2015.08.153. DOI: https://doi.org/10.1016/j.scitotenv.2015.08.153
Rengel M, Gil F, Montaño J. Crecimiento y dinámica de acumulación de nutrientes en caña de azúcar. Bioagro. 2011;23(2):135-40.
Sanchez AL, Arenas V, Marino EN, Dendooven L, Velazquez JB, Davila G, Rodríguez J, Hernández L, Contreras SM. Vinasse irrigation: effects on soil fertility and arbuscular mycorrhizal fungi population. J Soils Sediments. 2018;18(11):3256-70. DOI: https://doi.org/10.1007/s11368-018-1996-1
Senatore D, Quirolo A, Wajswol S, Bajsa N. Monitoreo de la aplicación de vinaza como fertilizante en caña de azúcar con indicadores microbianos del suelo. INNOTEC. 2017;13:92-7. DOI: https://doi.org/10.26461/13.09
Soobadar A. Application of vinasse to sugarcane. In: Webb E, editor. Sugarcane: production, consumption & agricultural management systems. New York: Nova Science Publishers; 2014. p. 329-58.
Vicente TF, Pedrosa EM, Rolim MM, Oliveira VS, Oliveira AKS, Souza AM. Relações de atributos do solo e estabilidade de agregados em canaviais com e sem vinhaça. Rev Bras Engen Agri Amb. 2012;16(11):1215-22. DOI: https://doi.org/10.1590/S1415-43662012001100010
Weih M, Hamnér K, Pourazari F. Analyzing plant nutrient uptake and utilization efficiencies: comparison between crops and approaches. Plant Soil. 2018;430(1-2):7-21. DOI: https://doi.org/10.1007/s11104-018-3738-y
Wilkie AC, Riedesel KJ, Owens JM. Stillage characterization and anaerobic treatment of ethanol stillage from conventional and cellulosic feedstocks. Biom Bioener. 2000;19(2):63-102. DOI: https://doi.org/10.1016/S0961-9534(00)00017-9
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Agrociencia Uruguay

This work is licensed under a Creative Commons Attribution 4.0 International License.
| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |